Targeted β₂-adrenergic signaling
Atrogi’s technology platform, building upon the idea of stimulating the β2-adrenergic receptor in a novel way, is designed to target a range of metabolic disorders, including diabetes, obesity, and muscle-wasting conditions.
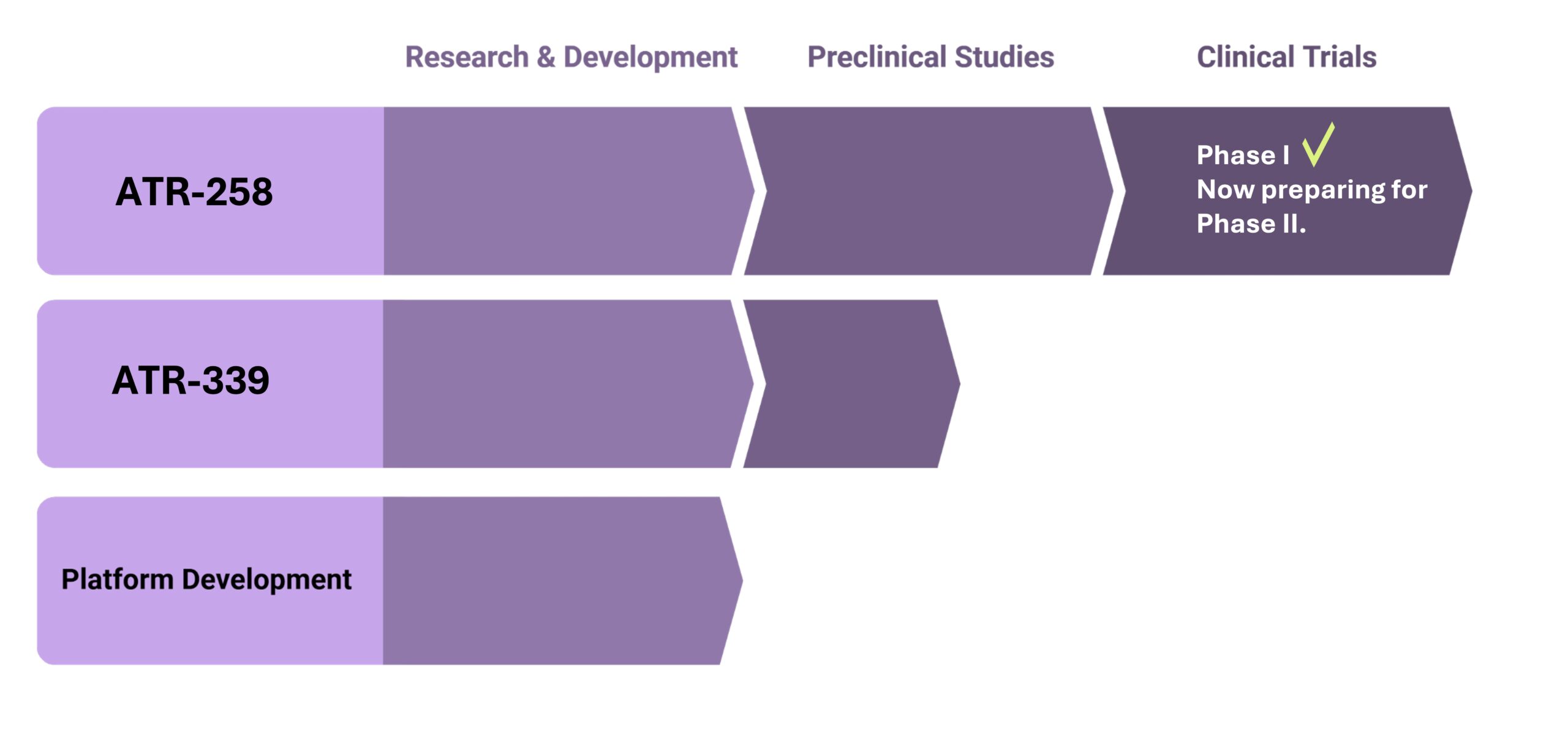
- ATR-258, has completed a successful Phase I safety study in both healthy volunteers and individuals with type 2 diabetes. Read more here!
- Ready for phase II.
- Once-daily, oral therapy designed to provide substantial advantages in both patient adherence and convenience over injectable therapies.
Additionally, its straightforward, scalable formulation supports a lower cost of goods, making it not only a patient-friendly option but also a commercially viable solution.
With proprietary compounds currently in preclinical testing for obesity and muscular wasting, we are committed to transforming the way these conditions are treated.
Diabetes is a growing global epidemic. Already, over half a billion people are living with diabetes worldwide, which is over 10.5% of the world’s adult population, and around 1.5 million deaths are directly attributed to diabetes each year1, 2.
With diabetes being a major burden on global healthcare systems, the world needs a revolutionary new solution that is effective, reliable, and safe, that can help patients have a better quality of life.
Type 2 Diabetes consists of a stepwise progression, starting with loss of muscle function, resulting in insulin resistance. Our lead asset, ATR-258, addresses the root of the problem, namely muscular insulin sensitivity, and has successfully completed a Phase I trial in healthy volunteers (n=48) and type 2 diabetes patients (n=24). Phase 2 clinical trials with ATR-258 is planned to start soon.
Obesity isn’t just a growing epidemic—it’s a global health crisis projected to affect over half the world’s population by 2035. At its core, obesity results from an imbalance in metabolism and the impact is profound, not only on individuals’ health but also on the financial strain placed on healthcare systems worldwide. The need for innovative, effective solutions has never been greater.
At Atrogi we are pioneering a revolutionary approach to obesity treatment. Our cutting-edge pipeline focuses on metabolic disorders, with a novel drug candidate designed to restore proper metabolic function without the muscle atrophy often caused by conventional treatments. Currently in pre-clinical development, our small molecule therapy has shown promising potential to increase metabolism and promote fat loss, while preserving muscle mass, positioning it as a powerful new solution in the fight against obesity.
We are on track to selecting a lead candidate for further clinical development, advancing our commitment to innovative and effective treatments for affected patients.