Stockholm, Sweden 3 of November
Atrogi AB, a phase II ready biotech company developing next generation oral muscle metabolism and obesity therapeutics, today announced the appointment of Dr. Paul Little as its new Chief Executive Officer, effective immediately.
Paul Little brings over 25 years of international experience spanning drug discovery, clinical development, and biotech investment. He previously served as CEO of Vesper Bio, where he successfully advanced programs from discovery to clinical Phase II, and as Operating Partner at the Lundbeck Foundation, supporting and building high-impact life science ventures. He also held board positions at several innovative biotech companies, including Folium.
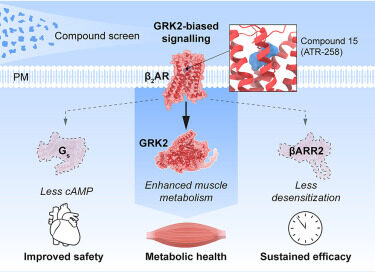
Atrogi is currently preparing for its next stage of clinical and corporate development, advancing its lead candidate ATR-258, an orally available β₂-receptor agonist designed to improve metabolism while preserving muscle mass. With Phase I data published in Cell and a strong proprietary pipeline, Atrogi is now well positioned to lead a new generation of metabolic therapies that go beyond GLP-1 analogues.
“Paul’s proven leadership, deep scientific background, and experience scaling biotech companies from early discovery to clinical validation make him an ideal leader for Atrogi’s next phase,” said Anders Ekblom, Chair of the Board. “We are confident he will accelerate our path toward bringing transformative treatments in metabolic health to patients worldwide.”
“Atrogi has built a unique GPCR Discovery Platform with over 1,000 proprietary compounds designed to fine-tune β₂ signalling with minimal desensitization, enabling applications across muscle and metabolism-related diseases. A platform that holds the potential to fundamentally change how we treat metabolic and muscle-related diseases,” said Paul Little, CEO of Atrogi. “I’m thrilled to join the team at this pivotal moment. Our biology is validated, our assets are strong, and our path is clear. We are now executing an accelerated plan to advance ATR-258 into Phase II and to engage investors and partners to capture the full potential of our technology.”
About Atrogi
Atrogi is a phase II ready Stockholm-based biotech company focused on revolutionizing oral treatments for metabolic diseases. The company has developed a proprietary screening platform to identify and validate pharmaceutical candidates targeting key cell-signalling pathways, with several potential molecules showing promising data in treating conditions beyond metabolic disorders. Our research focus on selective stimulation of the β₂-adrenergic receptor, which improves metabolism in fat and skeletal muscle cells, without causing muscle loss or cardiovascular strain. This approach has led to the development of ATR-258, a first-in-class oral β₂-agonist now advancing toward phase II evaluation in patients.