- Atrogi’s breakthrough discovery enables chronic use of next-generation, highly selective β2-agonists for the first time in metabolic disease
- First-in-class oral therapy ATR-258 mimics the effects of exercise – driving fat loss, increasing muscle, and improving metabolism – with broad potential in obesity, diabetes, and age-related muscle loss
- ATR-258 is set to enter Phase 2 trials to confirm its ability to deliver exercise-like benefits, including fat loss, improved muscle strength, and better metabolic control, alone or in combination with GLP-1 therapies
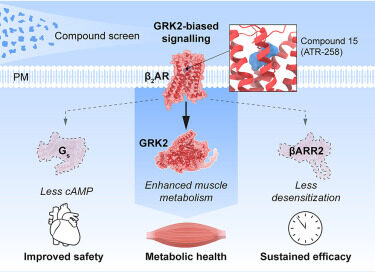
Stockholm, Sweden, June 23, 2025 – Atrogi AB, a clinical-stage biotechnology company pioneering muscle-targeted therapies, today announces the publication of a landmark study in the prestigious scientific journal Cell, titled “GRK-biased adrenergic agonists for the treatment of type 2 diabetes and obesity.” This paper highlights Atrogi’s first-in-class oral β2-adrenergic receptor agonist, ATR-258, which precisely activates skeletal muscle metabolism through novel GRK2-biased signaling. This transformative approach enhances glucose uptake and improves body composition by reducing fat and preserving lean muscle without the cardiovascular risks typically associated with β2-agonists.
“This marks a pivotal step in unlocking the full therapeutic potential of muscle-targeted β2-agonists,” said Professor Tore Bengtsson, senior author of the study, Chief Scientific Officer, and Founder of Atrogi. “Our research elucidates a novel signaling pathway that avoids the cardiac side effects of β2-agonists and provides compelling proof that ATR-258 can actively reshape body composition – reducing fat while preserving muscle mass – all without requiring dietary restriction. That’s an unprecedented therapeutic profile.”
Additionally, when combined with GLP-1 receptor agonists, ATR-258 avoids the critical shortcomings of current obesity and diabetes treatments by preventing the muscle loss typically associated with these therapies.
Associate Professor Morten Hostrup from the University of Copenhagen added: “This development offers patients a unique treatment option that does not require compromising muscle in the pursuit of weight loss. The potential applications of ATR-258 extend well beyond obesity and diabetes, encompassing a range of muscle-wasting conditions. By preserving muscle mass while supporting improved glucose regulation, ATR-258 may represent a significant advancement in therapeutic approaches across multiple clinical areas.”
The study will appear in the September 2025 issue of Cell and includes co-authors from multiple leading institutions across Europe and Australia. The breakthrough is the result of a long-term academic and industry collaboration led by Atrogi, together with scientists from Karolinska Institutet, Stockholm University, the University of Copenhagen, University of Nottingham, Monash University, and the University of Queensland. A key contributor was Assistant Professor Shane Wright of Karolinska Institutet, a leading expert in G protein–coupled receptor (GPCR) signaling, who co-elucidated the GRK2-biased mechanism that underpins ATR-258’s muscle-specific effects.
Key findings from the Cell study include:
- Development of GRK2-biased β2-agonists with potent effects on skeletal muscle metabolism and glucose uptake
- Prevention of muscle loss and improvement of metabolic health in preclinical models of type 2 diabetes, obesity, sarcopenia, and GLP-1–induced muscle wasting
- Favorable safety profile confirmed in long-term rodent and canine toxicology studies
- First-in-human trial data demonstrating safety and tolerability in both healthy volunteers and patients with type 2 diabetes
Alexandra Ekman Ryding, Chief Executive Officer of Atrogi, said: “We are excited by the findings of this paper which emphatically validate the potential of our novel compound to provide a disease-modifying treatment to those suffering with metabolic disorders. It is a testament to the drive, talent, and dedication of our world-class team that we are now Phase 2 ready. This proof-of-concept not only validates our approach but also demonstrates the strength of our proprietary discovery engine. It showcases what our broader platform is capable of – unlocking a pipeline of precision therapies that can address major unmet needs in metabolic and muscle-related diseases.”
In the near term, Atrogi is advancing ATR-258 into Phase 2 clinical trials, which will evaluate its ability to deliver exercise-like benefits, including fat loss, improved muscle strength and function, and better metabolic control – both as a monotherapy and in combination with GLP-1 receptor agonists.
For enquiries, please contact us here